Abstract
Background: Around 15% of children with acute lymphoblastic leukemia (ALL) experience disease relapse. The prognosis of these patients largely depends on the site of recurrence, time to relapse and leukemia immunophenotype. The ALL-REZ BFM 2002 and the ALL R3 trials have proved to be effective in previous trials. The IntReALL SR 2010 trial, conducted under the umbrella of the I-BFM SG, aimed at investigating feasibility, toxicity and efficacy of both treatment strategies in a large multi-national, multi-center prospective randomized trial.
Methods: Patients with a first standard-risk (SR) ALL relapse aged 1 - 17 years were recruited to the trial from 23/Oct/2014 to 31/Jul/2020 in 17 countries (210 centers). SR relapse criteria were: late (≥ 6 months after end of initial therapy) isolated bone marrow (BM) relapse, late or early (< 6 months after end of initial therapy and ≥ 18 months after initial diagnosis) combined BCP-ALL BM relapse, any late/early isolated extramedullary (IEM) relapse. They were randomized at a 1:1 rate to receive either arm A (ALL-REZ BFM 2002) or B (ALL R3). During consolidation, patients were randomized to receive the CD22-directed monoclonal antibody epratuzumab in addition to backbone chemotherapy. Results of this 2nd randomization will be communicated separately. Patients with late BM relapse were allocated to consolidation and maintenance chemotherapy or to allogeneic hematopoietic stem-cell transplantation (HSCT), depending on their minimal residual disease (MRD, cut-off arm A 10e-3 and arm B 10e-4) assessed after induction therapy. Patients with early either combined BM or IEM relapse were allocated to receive allogeneic HSCT.
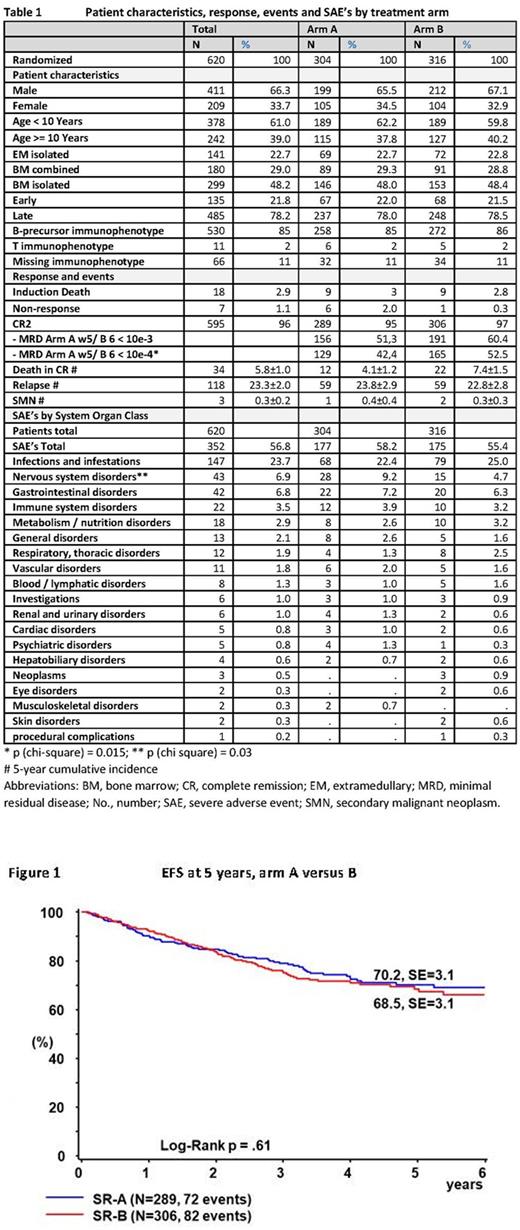
Results: Overall, 620 patients were randomized to receive arm A (n=304) or Arm B (n=316). Patient's characteristics were equally distributed among both groups (table 1). Induction death, 2nd remission rate, death in remission (5-years cumulative incidence (CI) 4.1 vs 7.4%, p=0.10), and subsequent relapse rates by arm A and B (CI 23.8 vs 22.8, p=0.80) did not differ between both groups. The MRD response rate with < 10e-4 post induction was significantly higher in Arm B (p=0.015), but not significantly different based on the arm specific cut-off, leading to comparable rates of allogeneic HSCT. The probability of 5-years event-free survival (pEFS) with arm A versus arm B was 70.2±3.1 SE versus 68.5±3.1 (p=0.61, figure 1) and that of overall survival (pOS) was 86.7±2.3 versus 87.1±2.0 (p=0.8). Subgroup analyses revealed a significantly higher pEFS in patients with isolated CNS relapse when treated with arm A (81.6±6.3, n=40) compared to arm B (43.3±8.3, n=45, p=0.001), whereas in patients with BM involvement or those with non-CNS IEM relapse, no significant difference was found (p=0.41). However, patients with isolated BM relapse experienced a higher CI of subsequent relapse with arm A (12.5±2.1, n=146) as compared to arm B (6.5±1.6, n=153, p=0.008). Patients with late BM relapse and MRD poor response after induction had comparable pDFS (arm A 65.8±6.4, n=74; arm B 68.8±6.2, n=75; p=0.71), which also did not differ from that of patients with MRD good response (arm A 67.7±5.6, n=100; arm B 68.9±5.6, n=113; p=0.77). Patients with late IEM relapse achieved a pEFS of 86.7±4.6 whereas those with early relapse and HSCT indication achieved a pEFS 61.8±5.4 (p=0.0004). Rates of reported severe adverse events (SAEs) per treatment phase did not differ between arms except nervous system disorders occurring more frequently in arm A (p=0.03, table 1).
Conclusion: Both arm A and B were effective in this large multi-national trial. EFS and OS probabilities compare favorably to results recently published by other cooperative groups. In particular, the outcome of patients with early IEM relapse or with late BM relapse and MRD poor response is encouraging confirming the benefit of allo HSCT in these subgroups. Due to superior outcome of isolated CNS relapse with arm A, patients without BM involvement will receive that arm as backbone in future trials. Since arm B showed a better MRD response rate after induction and a lower relapse rate in patients with BM relapse, it will be implemented as backbone for future trials in patients with BM involvement. Toxicity (TRM and SAE rates) is substantial in both arms demonstrating the urgent medical need of replacing toxic chemotherapy elements with equally effective, but less toxic targeted immunotherapy strategies in future trials.
Disclosures
von Stackelberg:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Speakers Bureau; Clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Attarbaschi:Amgen: Honoraria; Pfizer: Honoraria; Jazz: Honoraria; Takeda: Honoraria; Gilead: Honoraria; Novartis: Honoraria; MSD: Honoraria. Sramkova:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Duarte:Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lissat:Clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Baruchel:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Clinigen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Rizzari:Servier: Consultancy, Honoraria, Research Funding. Vinti:Merck & Co., Inc.: Research Funding; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Clinigen: Speakers Bureau. Locatelli:MEDAC: Speakers Bureau; GILEAD: Speakers Bureau; MILTENYI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANOFI: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NEOVII: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BLUEBIRD BIO: Speakers Bureau; TAKEDA: Speakers Bureau; SOBI: Speakers Bureau; PFIZER: Membership on an entity's Board of Directors or advisory committees; JAZZ PHARMACEUTICALS: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal